Characterization of non-covalent interactions
This paper tackles a fundamental question in chemistry: how to create and quantitatively characterize specific non-covalent interactions?
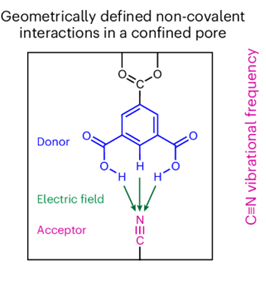
There is an enormous diversity of non-covalent interactions because such interactions can occur between any chemical groups that happen to get close enough. While certain types of non-covalent interactions, such as hydrogen bonds, have been well defined and characterized, a vast landscape of non-covalent interactions remain largely unexplored. To design and make non-covalent interactions, one must be able to put atoms in proximity without any direct covalent connection. In this manuscript we develop a general platform to systematically build non-covalent interactions by using a metal-organic framework (MOF) as a molecular scaffold to position selective chemical groups in defined geometry, combined with rigorous measurements of the electric fields associated with these interactions. Electric fields provide a universal and physics-based metric for these interactions. Using this platform, we discovered a series of non-covalent interactions with a wide range of electric fields. This includes fields as strong as those in enzyme active sites, to a field that is unusually destabilizing. We have engineered hydrogen bonds that are shorter and more head-on than those previously built in proteins, and created unique solvation under nanoconfinement, which exhibit electric fields distinct from those in bulk solutions.
Given that non-covalent interactions are central to chemistry, biology, and material science, we believe you will enjoy reading it:
Nature Chemistry 2025, https://doi.org/10.1038/s41557-025-01916-7