Understanding the Chemistry of Metal Oxide to Metal-Organic Framework Reactions for Morphology Control
At the beginning of this millennium, the advent of reticular chemistry sparked a breakthrough in the design and construction of porous crystalline solids with molecular precision. While this approach allows the design of crystalline porous metal-organic frameworks (MOFs) at the molecular level, a rational control over the 3D crystal morphology has not yet been achieved. Even the formation of simple three-dimensional structures, such as nanowires, is complicated and strongly dependent on the influence of external factors and usually cannot be realized by self-assembly approaches. However, this structural control is of great interest in order to efficiently apply MOFs for their diverse applications, ranging from catalysis and separation to sensing and drug delivery.
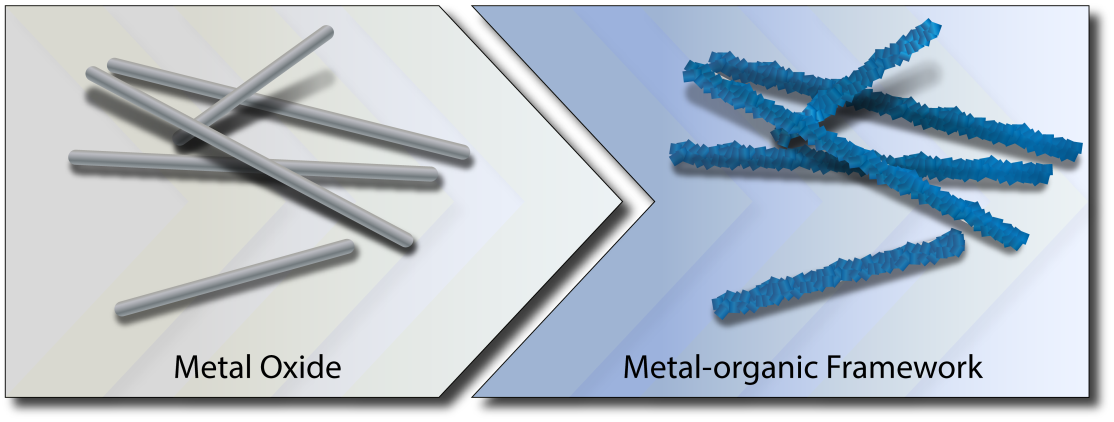
In this article, we propose a solution for overcoming the challenges of three-dimensional morphological control of MOF materials by providing structural control already through the selection of suitable metal oxide precursors serving as templates (see Figure). We apply this concept to various metal oxides and analyze in detail the underlying chemistry of the conversion reactions with zinc oxide as exemplary metal oxide, highlighting the influence of different reaction parameters on the reaction kinetics. Finally, we demonstrate the potential of this approach by using various complex three-dimensional zinc oxide structures as templates for the generation of MOF or metal oxide-metal-organic framework composite structures.
The central motivation of this work was to investigate two hypotheses: (i) If the reaction rates of the conversion of metal oxide to MOF are carefully controlled, shape-preservation of the metal oxide precursor is possible; and (ii) the exceptional and unusual reaction conditions used for the conversion of metal oxide to MOF can lead to new products and thus expand the landscape of MOFs structures. We were able to demonstrate the validity of the first hypothesis by studying in details the mechanistic specifics for the conversion of zinc oxide nanowires to ZIF-8 under different reaction conditions and to use the gained understanding for the preservation of different complex three-dimensional morphologies. Further, we have screened a number of different metal oxides to show the diverse applicability of our synthetic approach and further showcase its potential, by confirming the second hypothesis.
Please have a look:
Chemistry of Materials 2023, DOI: 10.1021/acs.chemmater.2c02946