Human Frontier Science Program Grant for modelling the electric field in enzyme catalysis
An international and interdisciplinary team of scientists, comprising Stefan Wuttke from BCMaterials (Spain) and Steven Boxer from Stanford University (USA), has received a prestigious three-year Human Frontier Science Program Research (HFSP) Grant. The project aims to study and understand the physical basis of enzyme catalysis.
Enzymes are remarkable biological catalysts responsible for driving complex metabolic reactions to occur at rates fast enough to sustain life. What happens inside an enzyme’s active site to allow slow and difficult chemical reactions to occur so rapidly? This remains an open question and has occupied biochemists’ attention for a long time. The textbook view of enzyme catalysis is depicted in Figure which shows a simple reaction coordinate from starting material, through a transition state, to product. The relative lowering of the transition state free energy is responsible for catalysis, but the origin of this lowering is rarely clear. Although enormous efforts have been made to determine enzymes’ mechanisms of action (i.e. the set of intermediates traversed en route from substrates to products), something essential is missing because the chemical mechanism an enzyme utilizes can be identical to the path taken in water, and yet the same process in the enzyme can be as much as 24 orders of magnitude faster.
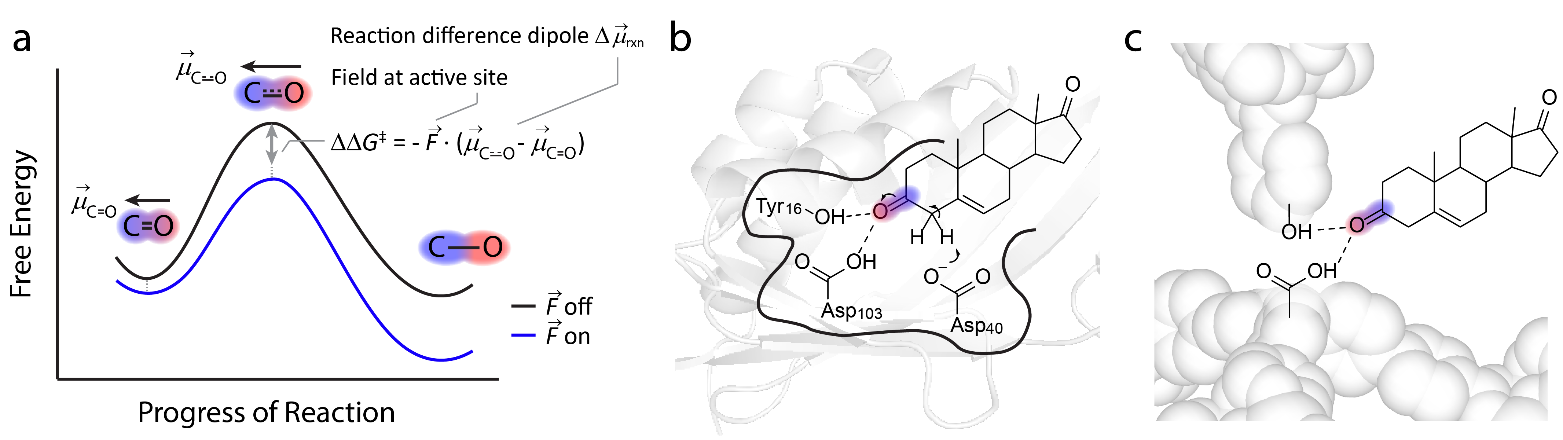
Figure. Electrostatic catalysis depicted along reaction coordinate, found in naturally occurring enzymes. a) Hypothetical effect of an electric field on a reaction pathway taking a less polar reactant through a more polar transition state illustrating electrostatic catalysis. b) The active site of ketosteroid isomerase exerts large electric fields on the C=O of the ketosteroid substrate through two short H-bonds.
One proposed contribution to reaction acceleration is the protein-exerted electric field for transition state stabilization, which has only recently been evaluated experimentally. The structures of enzymes have evolved to organize charges and dipoles into distinct configurations that can maximize the electric field projected to specific regions of their bound substrates. The funded project seeks to reconstruct enzymatic machinery and reproduce the extreme electric fields present in active sites using state-of-the-art supramolecular chemistry.
The Wuttke and Boxer lab are highly motivated to bridge the gap between the chemistry of living systems and classical synthesis in order to deepen the understanding of enzymes from a reductionist view. This project has received very positive reviewer feedback as only the top 4% of applications are funded. HFSP is a unique funding program that promotes international collaboration in basic research focused on the elucidation of sophisticated and complex mechanisms of living organisms. The team of Stefan Wuttke (BCMaterials), the lead principal investigator, and Steven Boxer (Stanford University) receives 280,000 USD per year for three years to carry out their innovative and cutting-edge project.


